IDENTIFICATION OF AAV SEROTYPES AND CHARACTERISATION OF THEIR STABILITY
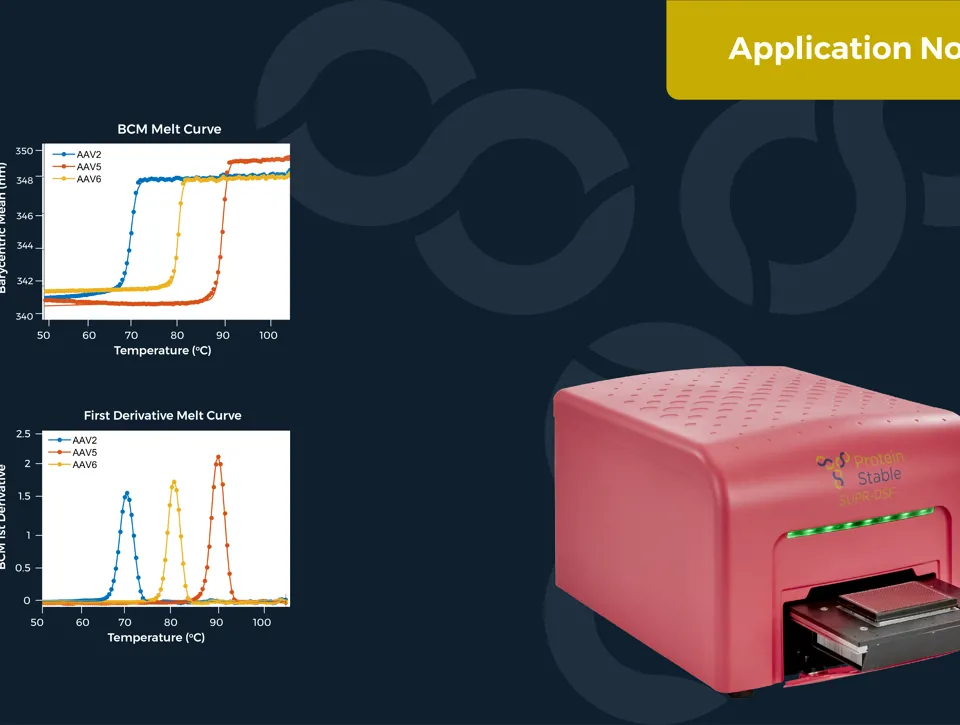
Measuring the thermal stability of three AAV serotypes, AAV2, AAV5, and AAV6, using the easy plate reader technology for protein stability screen: the SUPR-DSF. The SUPR-DSF measured the intrinsic fluorescence of each serotype during a thermal ramp to generate melt curves. In addition, we performed a concentration dilution series on AAV2 and AAV6 to determine the minimal sample requirements. All samples were performed at a well volume of 10μL in triplicate on a single 384-well microplate, emphasising the high-throughput possibilities of the instrument.










