Finding Formulations Faster with SUPR-DSF
Introduction
Successful formulation of biotherapeutic antibodies is fundamental to ensuring a stable and safe product that can withstand manufacture, storage, and transportation. This development is challenging because it requires a high throughput technique to measure the effects of hundreds to thousands of different formulation conditions. In addition, it is a time-consuming process creating a wide range of formulations and analysing the effects of each condition.
Using the SUPR-DSF with a pre-made formulation plate addresses both of those issues. The SUPR‑DSF can reliably measure thermal denaturation curves via intrinsic fluorescence in commercially available 384-well microplates. This allows you to compare a wide range of formulation conditions in a single experiment. Using this technology with the FORMOscreen® plate, which contains 96 ready-to-use buffers from formulations of developed antibodies approved by the FDA or EMA, allows you to obtain a single formulation quickly and easily for your therapeutic.[ 1]
In this application note we show the analysis of a thermal denaturation-based formulation screen of the commercially available therapeutic antibody Infliximab with the FORMOscreen® plate and SUPR-DSF instrument. This screen was measured in triplicate in a single 384‑well microplate in less than 2 hours. The barycentric mean (BCM) thermal denaturation curves were processed with a first derivative to find melting temperatures, which allowed us to come up with a single formulation that can be used with the antibody for future studies.
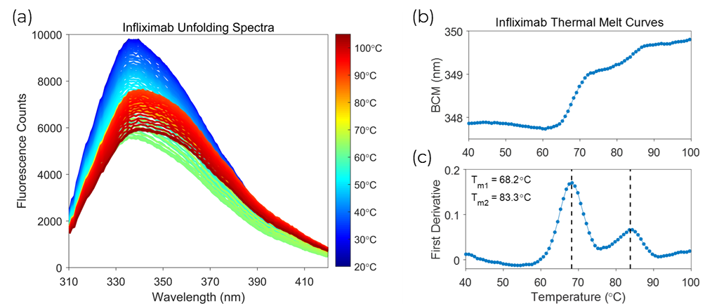
Figure 1 – (a) Fluorescence waveforms of Infliximab at each recorded temperature during the thermal ramp. (b) Barycentric Mean (BCM) melt curve. (c) First derivative melt curve.
Analysis
The SUPR-DSF measures the intrinsic fluorescence of proteins, primarily from the Tryptophan or Tyrosine residues. Analysing the fluorescence changes as a function of temperature allows you to quantify protein stability by computing melting temperatures of the proteins. This technique is very valuable in ranking the stability effects from many conditions.
The fluorescence waveforms generated in a thermal melt for the Infliximab control sample are shown in Figure 1(a). Processing the waveforms with the BCM allows you to analyse the wavelength shifts of the protein as it unfolds. The BCM thermal melt curve computed from the Infliximab spectra is shown in Figure 1(b). The first derivative of the BCM melt curve was processed to identify the melting temperatures. The first derivative analysis illustrated in Figure 1(c) shows two distinct transitions at 68°C and 83°C, which matched differential scanning calorimetry (DSC) results obtained by a collaborator.[2] All of the formulations in the screen were compared against the control sample to determine which formulation provides the highest amount of stability.
Results
The melting temperatures for all formulations, except ones under patent protection, are shown in Figure 2(a). Within this wide range of formulations, both increased and decreased stability of Infliximab was observed. The formulations in the upper right-hand corner increased the stability of both the first transition and second transition. The remaining formulations in other sectors of the plot either stabilised one or the other transitions or destabilised both. The formulation screen found the optimal buffer for Infliximab to be a salt-based buffer at a pH of 7.2, which is shown as a red circle in Figure 2(a). This formulation increased the melting temperature of the first transition by 2.3°C and the second transition by 1.3°C compared to the control sample. There were other formulations that had a larger stability effect on the second transition, but in most cases, it is best to pick the formulation that had the largest positive effect on the first transition because this is the domain that will unfold first under normal operating conditions.
The optimal formulation is composed of 8.5 mg⁄mL sodium chloride, 100 mg⁄mL maltose monohydrate, 1 mg⁄mL potassium hydroxide, and 1 mg⁄mL sodium phosphate monobasic dihydrate. The normalized fit curves for the first derivative of the optimal buffer compared to the control sample are shown in Figure 2(b). This figure clearly illustrates the stability increase with the top stabiliser shifting the peaks to higher temperatures for both transitions.
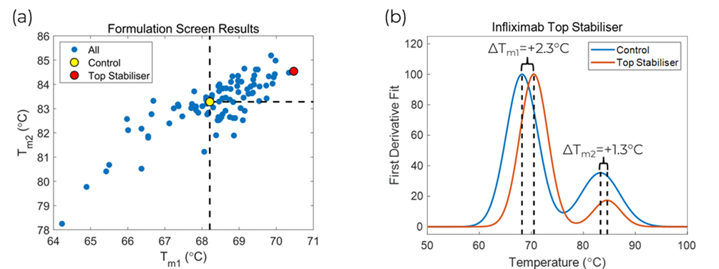
Figure 2 – (a) Scatter plot of fitted melting temperatures for all formulations. (b) First derivative fit plot of the control sample versus the top stabiliser found in the formulation screen.
Conclusion
The SUPR-DSF from Protein Stable demonstrates high data quality, capable of quantifying multi‑domain unfolding events of the therapeutic antibody Infliximab during a formulation screen in a single 2-hour experiment. In this formulation experiment, we determined the optimal formulation for Infliximab to be a salt-based buffer with a pH of 7.2. This formulation improved the stability of Infliximab by increasing the melting temperature of the first transition by a 2.3°C and the second transition by 1.3°C.
Pairing the easy connectivity and high-throughput capabilities of the SUPR-DSF with a pre-made formulation plate allows you to determine the optimal formulation for your target in a single experiment. This pairing greatly reduces the time it takes to complete formulation development and allows you to proceed to the next steps in your pipeline quickly and confidently.
Methodology
Microplate Preparation
The assay samples were made in a 96-well microplate by mixing 10 µL of stock FORMOscreen® formulations with 33.75 µL water, and 6.25 µL of stock Infliximab. The samples were mixed and transferred to a 384-well microplate with a 12-channel automatic pipettor in triplicate at a well volume of 10 µL. Infliximab was prepared at a final concentration of 1 mg⁄mL and the stock formulations were diluted by 5x. After dispensing, the plate was centrifuged for 60 seconds in a microplate centrifuge and sealed with an optically clear adhesive film. The plate was incubated at room temperature for 1-hour before measurement to allow for the protein to stabilise in the formulations.
Thermal Denaturation Measurement
The SUPR-DSF was set-up to measure the fluorescence spectra of Infliximab samples from 20°C to 105°C with a 1°C per minute ramp rate. Quantification of the spectral shift, that is indicative of protein denaturation, was determined via the barycentric mean (BCM) calculation. The BCM data was analysed as a function of temperature and the first derivative was determined from the BCM melt curve. Melting temperatures were calculated from the peaks of the derivative. The best formulation was determined by selecting the sample with the highest first transition melting temperature.
Methods and results for all samples are available on request.
References
[ 1] – FORMOscreen® Antibody Formulation Screen (5x stock). Developed by 2bind molecular interactions and produced by Jena Biosciences. 2bind Gmbh, Regensburg, Germany. Catalog # CS-360.
[2] – We thank Jeff Schwinefus’s laboratory (St. Olaf College, Northfield, MN) for the DSC results
