SUPR-DSF
Next Level Differential Scanning Fluorimetry
The SUPR-DSF by Protein Stable delivers next level differential scanning fluorimetry by simplifying sample preparation and analysis of protein stability into a single microplate.
Utilising precision optical components to deliver the highest sensitivity, intrinsic fluorescence of proteins and full spectrum detection, both thermal ramping and isothermal chemical denaturation experiments can be easily run in 384-well microplates.
Publications
Technology
- High throughput thermal ramping stability screening
- Intrinsic fluorescence - broad compatibility with biological buffers
- Fast, 384-well plates scanned at 1oC per minute
- Wide dynamic range of sample concentrations
- Very low protein requirement and volumes needed
Applications
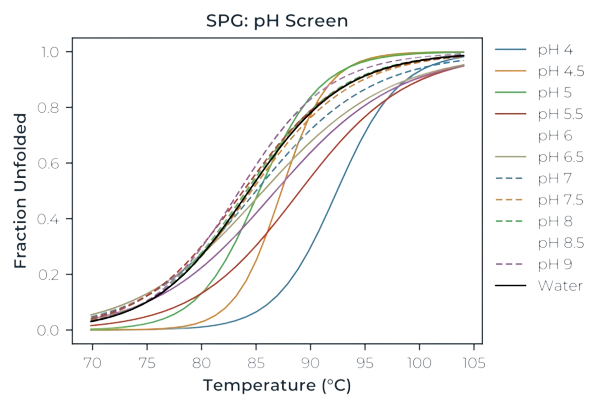
- Formulation and buffer optimisation
- Protein characterisation
- Stability profiling
- Similarity assessment
- Accelerated stress and forced degradation studies
- Binding-induced conformational changes analysis
- Post-translational modification assessment
Features
Next generation differential scanning flourimetry
- The SUPR-DSF was designed specifically to deliver great protein stability screening whilst simplifying processes, thus reducing operator time in preparing samples, reducing the amount and cost of consumables required, and reducing the risk of errors in extra preparation steps.
- By developing a system that can read directly in-plate, the shift in intrinsic fluorescence of protein samples as they unfold, Protein Stable has eliminated several steps seen in other techniques.
- Thus sample preparation starts and stops in a single 384-well plate and once loaded onto the thermal block that moves out of the drawer at the front of the instrument, it is simply a matter of loading up a method and starting a run in the graphical SUPR-Suite software. Once complete, the analysis and export of the data are a few clicks away.
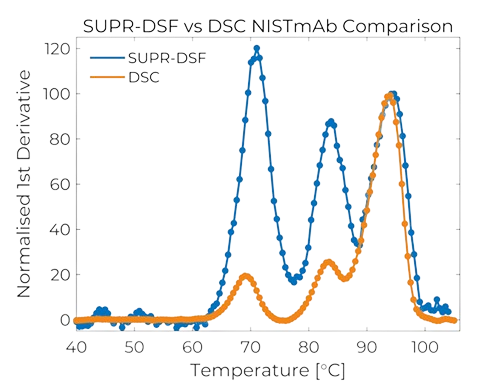
- Protein stability screens can reveal significant differences between samples’ melting temperatures, however the SUPR-DSF can be used to spot much smaller differences and more.
- Using a spectrometer to detect full emission of the intrinsic tryptophan and tyrosine residues far greater detail can be elucidated.
- Analysis options include several methods using either more traditional ratio methods of two wavelengths or more comprehensive methods using the spectrum. More data facilitates better signal to noise and more detail. On board automatic analysis breezes through 384 samples and should you wish to use external programs as preference, exporting data is straightforward.
- Preparing samples and subsequently analysing them in the same microplate certainly reduces the number of steps in the stability screening method, thus reducing operator interaction and risk of error, however the real efficiencies are realised in running costs.
- All you need to run the SUPR-DSF is a standard black 384-well PCR plate and a plate seal. No extra vessels to load the samples into, no cartridges or structures to hold them in place. The extras can be significantly expensive.
- Additionally, using a PCR plate allows sample volumes to be as little as 10 μL and with protein concentrations also being low, savings also extend to the sample you need to prepare.
Customer Experiences
See what our customers are saying about us!
Ellen Carrick
Ellen Carrick, a PhD student at the University of Bristol's Department of Physics, focuses on the study of soft matter, particularly the structural and colloidal stability of proteins. Her research has broad biological implications, including understanding cellular organization and disease pathways such as Alzheimer's.
Dr Jennifer McManus
Dr Jennifer McManus, Head of Physics at the University of Bristol, focuses on protein self-assembly, exploring how and why proteins associate in different contexts.
News & Events
