
01
Hidden Costs of NanoDSF Ownership
How much do you spend on your protein stability analysis?
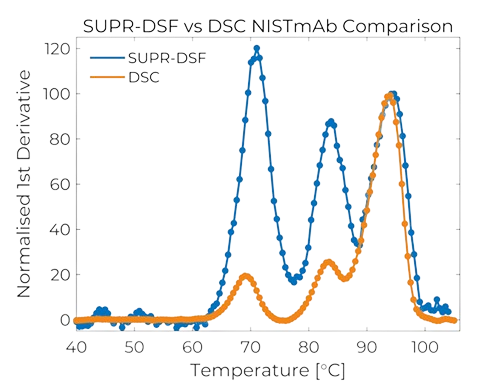
Next Level Differential Scanning Fluorimetry
The SUPR-DSF by Protein Stable delivers next level differential scanning fluorimetry by simplifying sample preparation and analysis of protein stability into a single microplate.
Utilising precision optical components to deliver the highest sensitivity, intrinsic fluorescence of proteins and full spectrum detection, both thermal ramping and isothermal chemical denaturation experiments can be easily run in 384-well microplates.
Ensure reliability with our five-year warranty, providing extended protection and peace of mind. This additional coverage ensures long-term support, safeguarding your investment. Please note that for the warranty to be valid, you need an active support plan.

01
How much do you spend on your protein stability analysis?

02

03
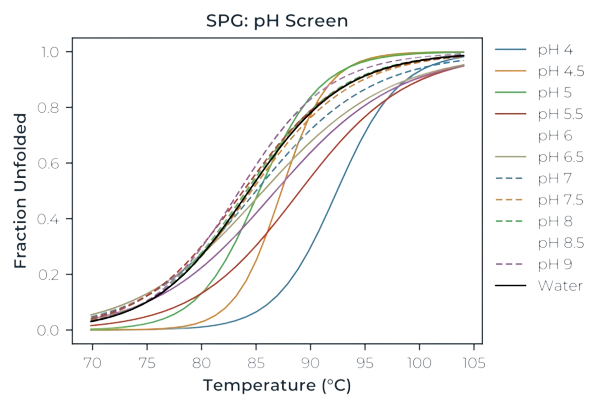
Next generation differential scanning flourimetry
Ellen Carrick, a PhD student at the University of Bristol's Department of Physics, focuses on the study of soft matter, particularly the structural and colloidal stability of proteins. Her research has broad biological implications, including understanding cellular organization and disease pathways such as Alzheimer's.
Dr Jennifer McManus, Head of Physics at the University of Bristol, focuses on protein self-assembly, exploring how and why proteins associate in different contexts.