Far-UV CD Analysis During Biotherapeutic Development: A Method Capability Study
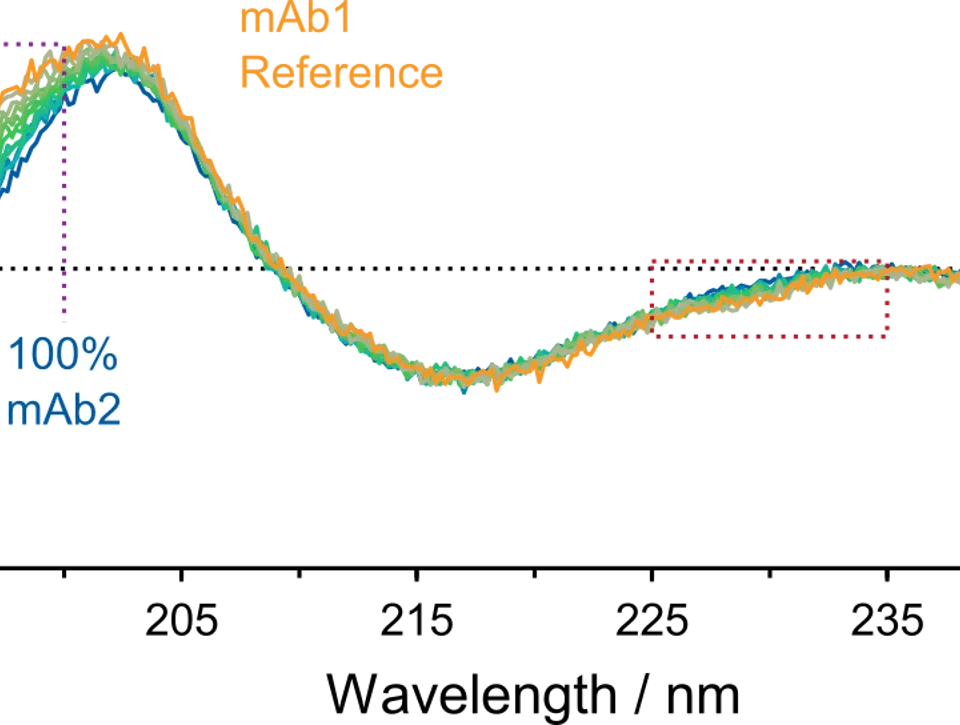
In this study carried out by Novartis, far- UV CD spectroscopy was qualified for the early development of a monoclonal antibody therapeutic. The high sensitivity of Chirascan V100 enabled low-level detection of an lgG1 mAb in mixture with a structurally high similar mAb. A demountable short-pathlength cell was used to minimize spectral contributions by formulation buffer excipients. Limit of Detection and Limit of Quantification was established, and CD data contributed to a successful biotherapeutic submission to regulatory authorities.



